
UGO Exoskeleton (Medical Use)
UGO is designed for patients with lower limb motor dysfunction resulting from spinal cord injury, lower limb myasthenia, or other neurological conditions. It is mainly used in hospitals’ rehabilitation departments and medical institutions to help with patients’ ambulatory pieces training.
Product Features

Safety and Effectiveness
Approved NMPA Certificates
Intent Detection
Multi-sensor Integration
Gait Regulation
Standard Gait Training

Bionic Design
Comply with Human’s Way of Movement

Cloud Data
Better Rehabilitation Assessment
Standard Gait Training
UGO provides repetitive and accurate walking training of high frequency, establishing normal gait and improving patients’ overall level of movement.

Full-cycle Rehabilitation
In addition to passive training which relies solely on equipment, a variety of active and active-passive-combined training modes are also available. Together with the online intelligent assessment, training programs can be tailored to suit different stages of rehabilitation.

Cloud-based User Management
We employ digital rehabilitation management. Cloud-based data management of users, institutions, and equipment facilitates intelligent rehabilitation and standardized records so that the programs are more scientific to help with training assessment and development.
Humanized Design
UGO is quick and comfortable to wear with its leg lengths electrically adjustable and its interactive video screen. Its mechanical fixation and strap protection effectively prevent patients from secondary injury.

Safety and Compliance
In addition to the Class II medical registration by the State Food and Drug Administration, the product has also received a series of international awards, including the iF Design, Red Dot, DIA, and Gold Dot, and has been awarded as an innovative medical device in the industry. The anti-misconduct module, emergency stop button, and removable support frame provide multiple safeguards.

Accessories
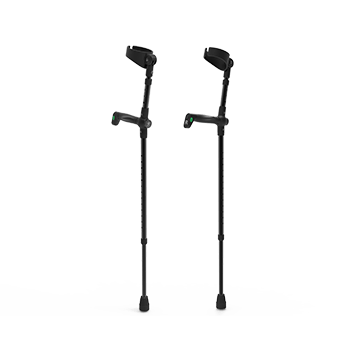 Smart Elbow Cane
Smart elbow canes can be used in conjunction with exoskeleton for support and assistance in walking training.
Smart Elbow Cane
Smart elbow canes can be used in conjunction with exoskeleton for support and assistance in walking training.
 Auxiliary Hanger
The hanger can support the arm and is suggested to be used together with the exoskeleton.
Auxiliary Hanger
The hanger can support the arm and is suggested to be used together with the exoskeleton.
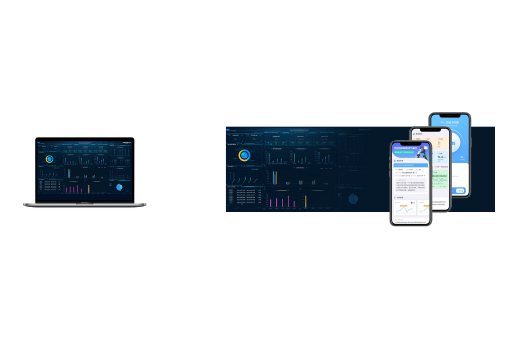
 Intelligent Training System
A software system equipped with UGO exoskeleton for rehabilitation training operations and data management.
Intelligent Training System
A software system equipped with UGO exoskeleton for rehabilitation training operations and data management.
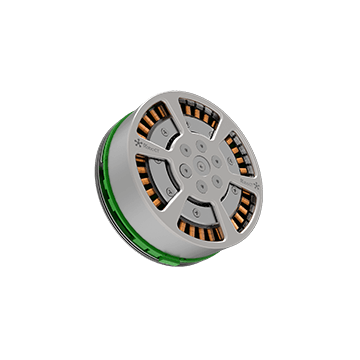 Joint Modules
The joint modules incorporate brushless servo motors, drives, gearboxes, encoders, and other micro-manipulation modules, which are adaptive for various articulated robots, bionic robots, exoskeleton robots, and other precision motion equipment. The modules are compact in size and powerful in momentum, providing considerable torque per unit with high efficiency at a low cost.
Joint Modules
The joint modules incorporate brushless servo motors, drives, gearboxes, encoders, and other micro-manipulation modules, which are adaptive for various articulated robots, bionic robots, exoskeleton robots, and other precision motion equipment. The modules are compact in size and powerful in momentum, providing considerable torque per unit with high efficiency at a low cost.
Scenarios
Q&As
Who is the UGO Exoskeleton for?
People with brain lesions, spinal cord injuries, or lower limb hypofunction caused by other central neuropathies. *The applicability of rehabilitation exoskeleton is subject to medical advice
What kind of medical device does UGO Exoskeleton belong to?
UGO, for all stages of rehabilitation, has obtained the registration certificate of Class II medical devices with the application scope of neuro-central pathologies (including paraplegia and hemiplegia).
What is the price of UGO Rehabilitation Exoskeleton?
The price of UGO Rehabilitation Exoskeleton (Medical Uasage) varies according to the partner hospitals in each region and the rehabilitation stages of the patient. Hospitals in some regions are covered by medical insurance. It is recommended to consult with rehabilitation medical institutions for details.
Where can I try UGO Rehabilitation Exoskeleton?
Partner hospitals of UGO are available on RoboCT’s website: http://www.roboct.com/order.

Recommendation

