Entering 2024 Top 100 Medical Device Company! RoboCT's Innovation in Rehab Exoskeletons
Release time:2024-05-15
Related article

From the 8th to the 10th, the 8th Future Medical Ecology Exhibition with the theme of "New Youth", hosted by VB100, Artery Network, Eggshell Research Institute, and Artery Orange, was grandly held in Beijing. During the conference, the 2024 China Future Healthcare Rankings was announced.
RoboCT Technology was announced as
2024 Top 100 Medical Device Company

RoboCT Technology focuses on the field of rehabilitation and elderly care. The five series of rehabilitation exoskeleton products independently developed and manufactured by RoboCT have obtained the NMPA registration certificate, filling the gap in the domestic market. RoboCT's rehab solution is designed for people with different lower-limb rehabilitation needs. Based on the principle of "neuroplasticity", it may accurately drive the wearer to carry out continuous and high-frequency rehabilitation training. All equipment is equipped with digital rehabilitation platform.
Taking advantage of cooperation with institutions, since 2023, RoboCT has comprehensively expanded the C-end product matrix and launched the UGO series, KidGo series, walking assistance exoskeletons and personal assistance robots to create equipment suitable for public, community and household usage.
RoboCT's KidGo (Children’s Rehab Exoskeleton) was enlisted in
the 2024 Most Innovative and Creative Products in Healthcare

The 2024 Most Innovative and Creative Products in Healthcare is selected on the basis of the growth and annual performance of the four indicators, including of technological innovation, clinical application, marketization, and industry influence. It is mainly selected from the four major fields: biological drugs and biological products, high-end intelligent manufacturing and medical devices, digital innovative technologies and supply chain enabling products. RoboCT's KidGo children's rehabilitation exoskeleton solution was among the list due to its advanced technology, efficient clinical application value, broad development space and deep industry influence.
Dr. WANG Tian,
founder and CEO of RoboCT Technology
conducted the Product Roadshow

During the conference, a launch conference was held for representative products. Dr. WANG Tian, founder and CEO of RoboCT Technology, gave a roadshow for KidGo exoskeleton. The product is based on the practicality of children's rehabilitation, which will greatly alleviate the situation of insufficient supply of rehabilitation resources, intervene during the growth and development period of children and the golden period of early rehabilitation, and make up for the shortcomings in the adaptability of traditional walking rehabilitation therapy. Through precise and uniform traction and active triggering mode, the rehabilitation experience of children is improved and the subjective initiative of recovering children is enhanced.
KidGo's children's exoskeleton has been approved for Class II medical device certificate in October 2023. It is also the first children's exoskeleton product in the country to obtain an NMPA registration certificate. The continued popularity of the KidGo series of children's exoskeletons will bring new development for children's rehabilitation and help more children grow up healthily and happily.
2024 Future Medical Top 100 Growth Analysis Report
Case Study: RoboCT Technology

The "2024 Future Medical Top 100 Enterprises Growth Analysis Report" launched on the occasion of the Conference, conducted a comprehensive analysis of the five main list companies, summarized the corporate growth patterns and industry change trends from a multi-dimensional perspective. The report conducts a detailed case analysis of RoboCT Technology, and deeply explores the story behind its growth from aspects such as product R&D, marketing, and business models. It is also expected that this report can have a positive impact and promote the future development of the medical and health industry.
RoboCT Technology launched classic series of rehabilitation exoskeleton
in 2024 VBEF Exhibition Area A39

During the same period of the conference, RoboCT participated in the new product exhibition area of the VBEF Ecological Exhibition, visually displaying the latest series of rehabilitation exoskeleton. Exhibitors have a deeper understanding of the product appearance, performance, advantages and practicality. Through interactive communication with the on-site audience, the company further understood of market demand and industry dynamics, which provide reference and inspiration for future product development and marketing. In the near future, RoboCT Technology will continue to make greater breakthroughs and innovations in the field of rehab exoskeleton.
 Being Reported on CCTV-13 News Programme Zhao Wen Tian Xia ! Experiencing RoboCT Technology's Rehab Exokeleton!
2025-02-08
Being Reported on CCTV-13 News Programme Zhao Wen Tian Xia ! Experiencing RoboCT Technology's Rehab Exokeleton!
2025-02-08
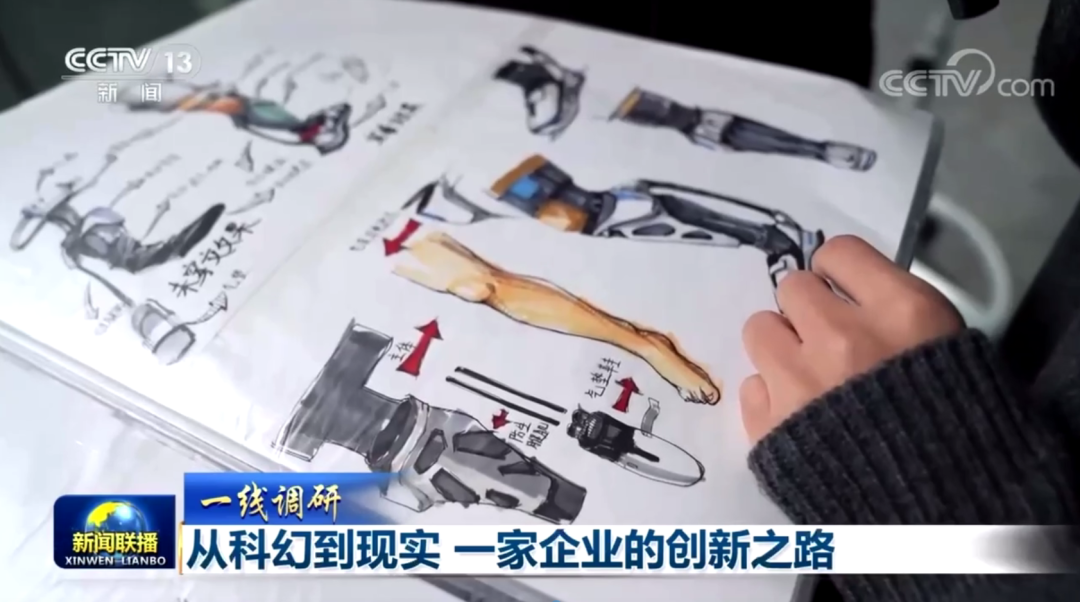 Exoskeletons are Hotly Discussed , and RoboCT has been Featured in CCTV News
2023-02-09
Exoskeletons are Hotly Discussed , and RoboCT has been Featured in CCTV News
2023-02-09
 RoboCT was Selected as One of the Experts and Typical Cases of Science &Technology Assistance for People with Disabilities
2025-11-07
RoboCT was Selected as One of the Experts and Typical Cases of Science &Technology Assistance for People with Disabilities
2025-11-07
 RoboCT's GoGo Exoskeleton Empowers the Double Ninth Festival Mountain Climbing Activity
2025-11-07
RoboCT's GoGo Exoskeleton Empowers the Double Ninth Festival Mountain Climbing Activity
2025-11-07
 To Build an Intelligent Healthcare Ecosystem, RoboCT Made a Stunning Appearance at the 2025 CR Expo!
2025-09-15
To Build an Intelligent Healthcare Ecosystem, RoboCT Made a Stunning Appearance at the 2025 CR Expo!
2025-09-15
 RoboCT's URA Multi-Pos Lower Limb Rehabilitation System Won the Bronze Award of the 2025 New Material Design Award
2025-09-05
RoboCT's URA Multi-Pos Lower Limb Rehabilitation System Won the Bronze Award of the 2025 New Material Design Award
2025-09-05
 Integration of Medical-care and Healthcare, RoboCT Takes the Lead in Tackling National Pilot Projects!
2025-09-05
Integration of Medical-care and Healthcare, RoboCT Takes the Lead in Tackling National Pilot Projects!
2025-09-05
 RoboCT's Exoskeleton Helps Explore a New Chapter in Illuminating the Future of Cities
2025-08-22
RoboCT's Exoskeleton Helps Explore a New Chapter in Illuminating the Future of Cities
2025-08-22
 The Healing Power of Rehab Exoskeletons at the Opening Ceremony of the CMG World Robot Skills Competition
2025-08-22
The Healing Power of Rehab Exoskeletons at the Opening Ceremony of the CMG World Robot Skills Competition
2025-08-22
 Smart Mountaineering Experience! Japan's Mount Fuji Scenic Area introduces RoboCT's GoGo-H Exoskeleton Robot
2025-08-22
Smart Mountaineering Experience! Japan's Mount Fuji Scenic Area introduces RoboCT's GoGo-H Exoskeleton Robot
2025-08-22