The 2023 National Medical Device Standard Revision Plan was Announced! The Medical Lower-extremity Exoskeleton was on the List
Release time:2023-06-09
Related article
Recently, the National Medical Products Administration released the "2023 Medical Device Standard Development and Revision Plan Projects List" (hereinafter referred to as the "Notice"). The recommended standard formulation and revision project plan will then be formulated and RoboCT will participate in it.
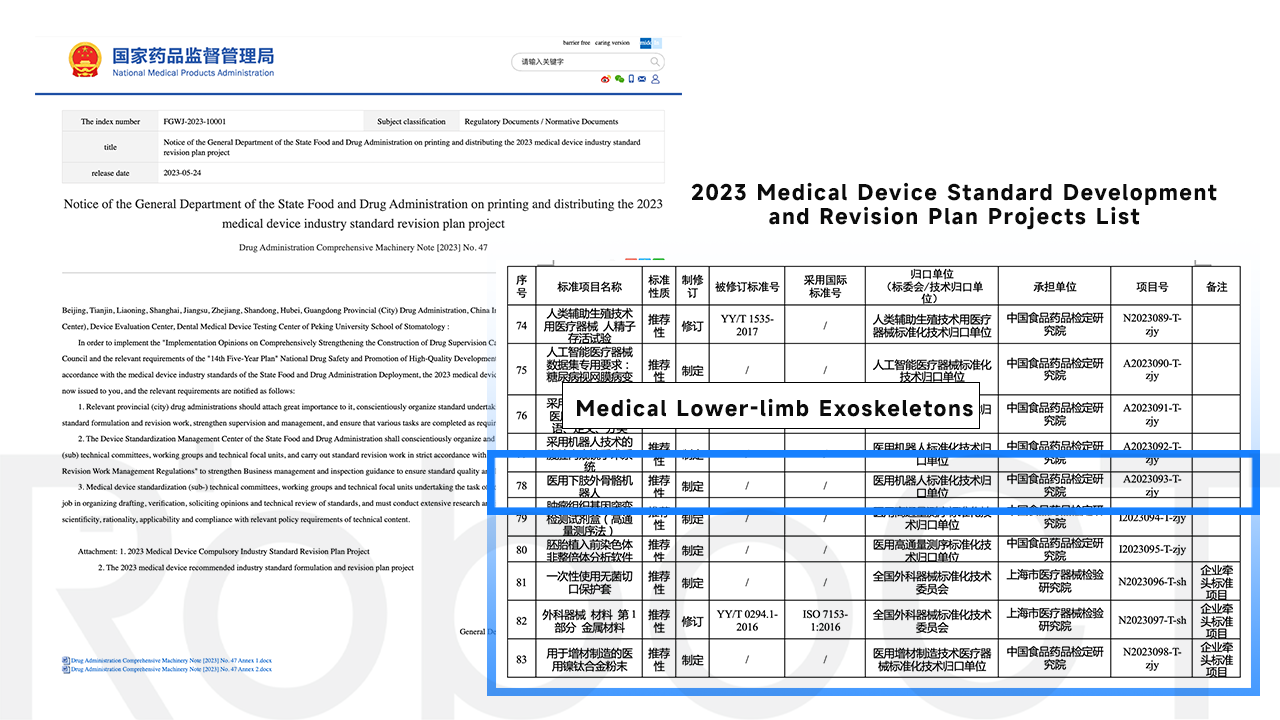
The standardization not only helps to improve the safety, effectiveness and reliability of medical devices, but also plays an important role in protecting the interests and rights of patients. With further implementation of the "Notice", China's medical device standards will form its own system to guide future development.
Formulation of the recommended industry standards for "Medical Lower-limb Exoskeletons" will promote its future market competition for companies like RoboCT, which focuses on lower-limb rehabilitation exoskeleton products and services. In the long run, it will also benefit the industry as a whole Increased concentration. RoboCT also hopes to contribute its corporate wisdom, value and experience to the overall technical development of the industry through active participation.
 Being Reported on CCTV-13 News Programme Zhao Wen Tian Xia ! Experiencing RoboCT Technology's Rehab Exokeleton!
2025-02-08
Being Reported on CCTV-13 News Programme Zhao Wen Tian Xia ! Experiencing RoboCT Technology's Rehab Exokeleton!
2025-02-08
 Exoskeletons are Hotly Discussed , and RoboCT has been Featured in CCTV News
2023-02-09
Exoskeletons are Hotly Discussed , and RoboCT has been Featured in CCTV News
2023-02-09
 RoboCT Technology's Rehab Exoskeleton was Exhibited at Arab Health 2025
2025-02-08
RoboCT Technology's Rehab Exoskeleton was Exhibited at Arab Health 2025
2025-02-08
 RoboCT Technology's Rehab Exoskeleton was Selected as Outstanding Case Study for “Sustainable Investment for the Future"
2025-01-10
RoboCT Technology's Rehab Exoskeleton was Selected as Outstanding Case Study for “Sustainable Investment for the Future"
2025-01-10
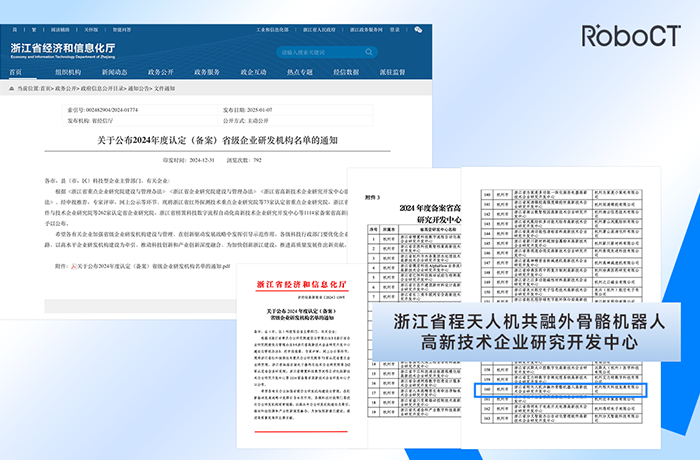 RoboCT Technology Has Been Selected as the 2024 Zhejiang High-tech Enterprise Research and Development Center!
2025-01-10
RoboCT Technology Has Been Selected as the 2024 Zhejiang High-tech Enterprise Research and Development Center!
2025-01-10
 RoboCT Technology's Exoskeleton Wins Product Masterpiece Award in the First “Better and Better” International Design Competition
2025-01-01
RoboCT Technology's Exoskeleton Wins Product Masterpiece Award in the First “Better and Better” International Design Competition
2025-01-01
 RoboCT Technology's Rehab Exoskeleton Wins Made in China Beauty Award of 2024
2025-01-01
RoboCT Technology's Rehab Exoskeleton Wins Made in China Beauty Award of 2024
2025-01-01
 Case Report of Time-Sequenced Spinal Cord Electrical Stimulation Combined with Exoskeleton Training was Published in ACTN Journal
2024-12-20
Case Report of Time-Sequenced Spinal Cord Electrical Stimulation Combined with Exoskeleton Training was Published in ACTN Journal
2024-12-20
 RoboCT Technology's Exoskeleton Won the 2nd Prize of the 2024 China Association of Automation Science and Technology Progress Award!
2024-12-20
RoboCT Technology's Exoskeleton Won the 2nd Prize of the 2024 China Association of Automation Science and Technology Progress Award!
2024-12-20
 RoboCT’s UGO Serie Rehab Exoskeleton Has Been Approved for CE Certification as a Medical Device
2024-12-13
RoboCT’s UGO Serie Rehab Exoskeleton Has Been Approved for CE Certification as a Medical Device
2024-12-13