The Industry Standard for Medical Exoskeleton, in which RoboCT Participated in, was Officially Released
Release time:2025-06-27
Related article

On the 26th, the National Medical Products Administration officially released 38 medical industry standards, of which the 30th "Medical Lower Limb Exoskeleton Robot" (YY/T 1973-2025) filled the gap in domestic powered medical exoskeleton standards. As one of the core drafting units, RoboCT was deeply involved in the formulation of this standard.
The standard focuses on the safety, reliability and performance requirements of rehab exoskeletons, covering key technical indicators such as operating performance, static load strength, fatigue testing, noise control, and functional safety. Compared with existing general specifications, the new standard emphasizes the characteristics of medical devices and provides an authoritative basis for product development, production and testing. The introduction of the standard clearly states that, on the premise of ensuring that risks are controllable, technological innovations are encouraged.
As one of the few private enterprises in the drafting unit, RoboCT Technology, with its technical accumulation in exoskeleton structure design, motion control algorithms and clinical applications, provides practical support for key clauses such as standard test methods and mechanical performance requirements. The company has joined hands with more than ten authoritative institutions such as the China Food and Drug Inspection Institute, Zhejiang University, and Tianjin University to jointly promote the integration of standards and industry needs, and help enhance the international competitiveness of domestic exoskeleton products.
 Being Reported on CCTV-13 News Programme Zhao Wen Tian Xia ! Experiencing RoboCT Technology's Rehab Exokeleton!
2025-02-08
Being Reported on CCTV-13 News Programme Zhao Wen Tian Xia ! Experiencing RoboCT Technology's Rehab Exokeleton!
2025-02-08
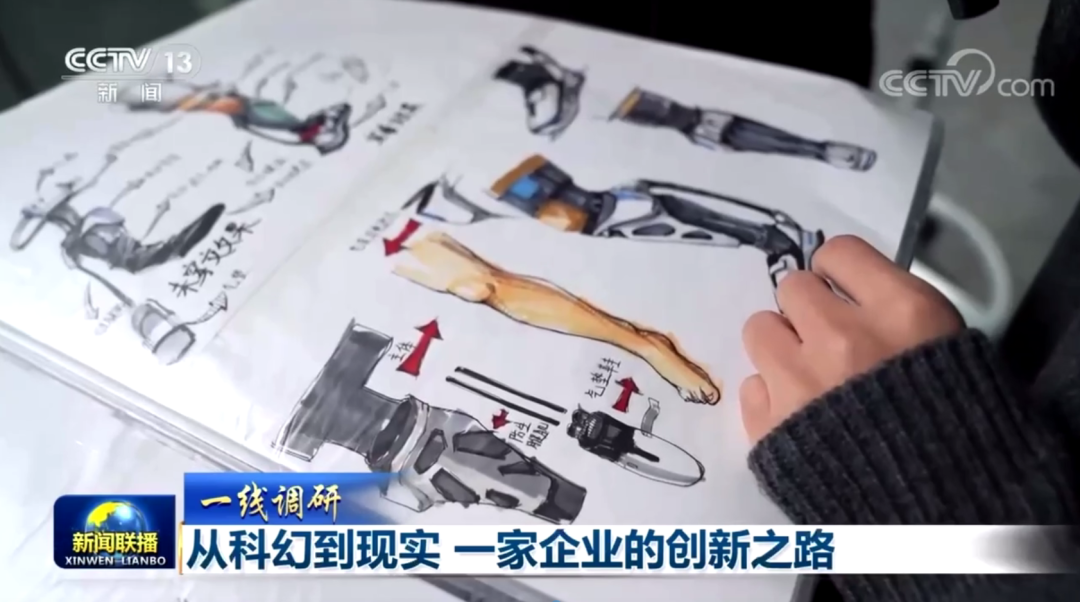 Exoskeletons are Hotly Discussed , and RoboCT has been Featured in CCTV News
2023-02-09
Exoskeletons are Hotly Discussed , and RoboCT has been Featured in CCTV News
2023-02-09
 RoboCT was Selected as One of the Experts and Typical Cases of Science &Technology Assistance for People with Disabilities
2025-11-07
RoboCT was Selected as One of the Experts and Typical Cases of Science &Technology Assistance for People with Disabilities
2025-11-07
 RoboCT's GoGo Exoskeleton Empowers the Double Ninth Festival Mountain Climbing Activity
2025-11-07
RoboCT's GoGo Exoskeleton Empowers the Double Ninth Festival Mountain Climbing Activity
2025-11-07
 To Build an Intelligent Healthcare Ecosystem, RoboCT Made a Stunning Appearance at the 2025 CR Expo!
2025-09-15
To Build an Intelligent Healthcare Ecosystem, RoboCT Made a Stunning Appearance at the 2025 CR Expo!
2025-09-15
 RoboCT's URA Multi-Pos Lower Limb Rehabilitation System Won the Bronze Award of the 2025 New Material Design Award
2025-09-05
RoboCT's URA Multi-Pos Lower Limb Rehabilitation System Won the Bronze Award of the 2025 New Material Design Award
2025-09-05
 Integration of Medical-care and Healthcare, RoboCT Takes the Lead in Tackling National Pilot Projects!
2025-09-05
Integration of Medical-care and Healthcare, RoboCT Takes the Lead in Tackling National Pilot Projects!
2025-09-05
 RoboCT's Exoskeleton Helps Explore a New Chapter in Illuminating the Future of Cities
2025-08-22
RoboCT's Exoskeleton Helps Explore a New Chapter in Illuminating the Future of Cities
2025-08-22
 The Healing Power of Rehab Exoskeletons at the Opening Ceremony of the CMG World Robot Skills Competition
2025-08-22
The Healing Power of Rehab Exoskeletons at the Opening Ceremony of the CMG World Robot Skills Competition
2025-08-22
 Smart Mountaineering Experience! Japan's Mount Fuji Scenic Area introduces RoboCT's GoGo-H Exoskeleton Robot
2025-08-22
Smart Mountaineering Experience! Japan's Mount Fuji Scenic Area introduces RoboCT's GoGo-H Exoskeleton Robot
2025-08-22